Thursday, October 25, 2007
Wednesday, October 24, 2007
Been Workin'
Over the past month or so, I've put a lot of effort into learning XHTML and CSS. I've also just begun the process of trying to wrap my head around AJAX. Why? To create a web-based homebrew calculator that doesn't rely on CGI, Perl or Flash. I want it to be as much straight-up CSS and Javascript as possible so that users won't require the latest plug-ins, load times will be fast, and the markup will validate.
To this end, I've already created what I believe will be the first official version of the BrewCalc site. It's barren as of yet, but that's because I've been working on the the design and layout for a number of weeks now. There's nothing sophisticated about it - it's quite stripped down. Now that the first portion is complete, I can begin working on creating the calculator interface. I anticipate it will take me a few months to complete at this rate, so the BrewCalc headquarters will simply be a place to download the current spreadsheet, tell others about it, and contact the author.
There are a lot of things I dream about doing with the online version. Outputting the results into a spreadsheet, or an XML file (ala BeerXML), generating graphs and charts, and being able to share the results with fellow brewers in a meaningful way are all ideas I'd like to incorporate. I also dream about creating an online community of brewers based around recipe formulation powered by Ruby on Rails. Of course, this is far in the future, but an aspiration nonetheless.
But enough daydreaming. I'll keep you posted on things as they progress.
As for the here and now, Hannah asked a few weeks ago if the perceived off-aroma that I had noticed in my last Golden Ale might be due to fusel production in bottle conditioning (at least, this is how I understood the question). To answer her first question, no, the aroma mentioned did not seem to be present pre-bottling. To answer her second question, it seems unlikely that fusels could be produced during the conditioning process as they are principally a result of yeast growth. However, all of the significant yeast growth ought to have occurred at this point. So, it seems improbable that fusel production could occur in the conditioning phase.
I'm still not sure as to the cause of this off-aroma. In fact, I'm still having difficulty discerning exactly what it is. I ought to have had some other experienced tasters done an analysis to see if there's something I'm missing. I suspect there is. I'll be sure to note if this occurs in later beers.
Be sure to let me know if you have any thoughts, or if any of my ideas about fusel production during the conditioning phase seem off. Also, let me know if you have any ideas for BrewCalc and the related projects I'd mentioned above.
Happy brewing!
Tuesday, October 9, 2007
Golden Ales
I find this surprising in that they're often thought of as beers that aren't terribly interesting. However, they are an excellent way to gauge one's brewing progress. If you can brew a tasty beer with one or two malts, one hop variety, and a fairly neutral yeast, I'd say you're well on your way to becoming a great brewer.
The last Golden Ale I brewed had a slight off-aroma that I'm still puzzled over. I feel as though I should be able to nail it on the head as I've gone through the BJCP exam twice now and have been judging and brewing for about two and a half years now. However, I'm still at a loss to describe it to my satisfaction. My best guess is that it's a fusel alcohol aroma that I had not come across before. It smells to me as I imagine fermented corn sugar would smell. I'm not sure precisely where I got this notion, but it's the one firmly implanted in my head.
All that being said, I think I'm going to make many more Golden Ales in the future. I don't have a signature beer, but I think with some work, this could be it. There could be a whole series of them. It would also serve as a great way to really get to know my base grains and hop varietals.
The first will be Maris Otter and homegrown Centennial. I'll update as things progress.
Saturday, October 6, 2007
BrewCalc + My Return
So far there are only two. They are entitled "Modify_PPG" and "Hide_PPG" respectively. They both do precisely what one might expect. Modify_PPG enables the user to access the column containing the pertinent Points Per Gallon info for the various grain types. Hide_PPG makes it invisible once again so that the screen is less cluttered with numerical data. Hooray for the power of customization!
I bottled a Foreign Extra Stout last night that has probably spent too much time in primary. Hopefully it renders itself reasonably drinkable within the coming months.
Lastly, here are some of the more noteworthy beers that I sampled while on the west coast. I'll try to write some more about them as I get a bit more time.
Krusovice (Czech Schwarzbier)
DeProef Reinehaerdt Wild Ale (Strong Golden-style w/Brettanomyces)
DeProef Saison Imperiale
Anchor Porter on hand pump
Russian River Pliny the Elder
Russian River Blind Pig
New Belgium 1554
Moonlight 60 to Life
Moonlight Death & Taxes
Russian River Perdition
Stone 11th Anniversary
Pizza Orgasmica IPA
Bear Republic Racer 5
SpeakEasy Double Daddy
Bear Republic Red Rocket
Tuesday, September 18, 2007
Out of Town
Cheers!
Thursday, September 13, 2007
Scale Recipes Up/Down
I'll probably be updating BrewCalc in the next week or so with some fun new stuff. In particular, I'm working on making the Points per Gallon numbers adjustable by the user without futzing a whole lot with the calculation in J13. I'll post once I get it up and running.
Wednesday, September 12, 2007
In case you ever wanted to do this backward...
Where,
O = Oz. of hops
I = IBUs
U = Utilization Factor (Bigness factor*Boil time factor)
G = Gallons of Finished Beer
A = Alpha Acid percentage
(O*A*U*74.9)/G = I
This is not the original formulation that Tinseth provides, but it produces the same numbers regardless.
If you had a target IBU in mind, and wanted to determine what amount you would need to reach it, here's the formula you would use:
I/[(A*U*74.9)/G] = O
Monday, September 10, 2007
Behind the Scenes
I've been working on a utility for BrewCalc that will enable the user to scale recipes up or down. It will even adjust amounts of your ingredients based upon extract efficiency. I have the grain portion of this sheet worked out, now I'm working on adding the hops portion. Here's how it will work when its finished: The user will enter a recipe that he/she has been given in the first worksheet ('OG, IBUs, ABV%, etc.'). The user will then click on the Scale Up/Down sheet where figures will be calculated based upon batch size, extract efficiency, and new hop utilization figures (as per Tinseth). The end result will be the new amounts for each ingredient listed in the original recipe.
Secondly, I've been putting together some new charts as of late. I've been working on graphing out similar styles on a graph whose Y-axis is Original Gravity and whose X-axis is IBUs. These graphs show ranges of OG/IBUs and even show where the intersections are of similar styles. I only have a few complete thus far, but I'll begin to post them as I continue to work on them.
Lastly, I just took the BJCP exam for the second time last night. It was as long and arduous as I recalled, though I suspect I bettered my score substantially this time around. I'll find out exactly how I fared in the next three to four months. That being said, I'm a little burnt out on technical homebrewing information right now, which means that Tech Tues will have to get pushed back to next week once again. Hope to see you then.
Wednesday, September 5, 2007
How Much Water Do I Add?
In contrast to the previous post about boosting OG by adding DME, this is going to show you how to calculate the amount of water you'll need to decrease OG.
(Note: This method assumes that you have already cooled your wort and are planning on adding water to the fermenter. Of course, you should never fill a bucket or carboy so much that you don't have at least a gallon or two or headspace left for the krausen. Additionally, it is advisable to add the cleanest water possible. This will lessen your chance of infection by bacteria or wild yeast that may be present in your water source. Boiling water and cooling it rapidly is a good method for making sure your top-off water is clean.)
You're almost to the end of your brewday. Your cooled wort is in the fermenter and you're taking a hydrometer reading for your records. Oh horror of horrors, you're ten points high! You know that you can dilute the wort you have to reach your desired OG, but how much water shoul you add?
Here are the numbers that we need at the outset:
1.) Gallons of Wort
2.) Original Gravity in GUs (pre-water addition)
3.) Desired original gravity (in GUs)
4.) Total Gravity Units [Original Gravity (in GUs)*Gallons of Wort]
(A quick word about GUs: You can convert specific gravity to GUs by multiplying OG*1000, then subtracting 1000.)
Let's say you have 5 gallons of 1.060 wort. However, you wanted an OG of 1.050. First find the Total Gravity Units (GUs).
[Original Gravity (in GUs)*Gallons of Wort] - 60*5 = 300.
Here's the calculation you'll use:
[Total Gravity Units/Desired OG (in GUs)] - Gallons of Wort. That's it!
[300/50] - 5
6-5 = 1 Gallon.
You'll require an additional gallon of water in order to bring your gravity down to the desired 1.050.
Here it is one more time with a new set of numbers.
1.) Gallons of Wort = 3
2.) Original Gravity in GUs (pre-water addition) = 77
3.) Desired original gravity (in GUs) = 54
4.) Total Gravity Units [Original Gravity (in GUs)*Gallons of Wort] = 231
[231/54] - 3
4.28 - 3 = 1.28 Gallons.
Sunday, September 2, 2007
DME Calculations
This is really easy. Daniels[1] tells us that we can reasonably expect 45 points per gallon from DME. That means that if we dissolved 1 pound of DME in one gallon of water, we'd have a resulting OG of 1.045. So to make a five galllon batch with this same OG, we'd need five pounds.
Do this first. Divide 45 by the size of your batch in gallons. I typically do five gallon batches, so 45/5 = 9. This number just tells me how many points I can expect to yield when adding 1 pound of DME to a five galllon batch. Makes sense too - add five pounds to five gallons at 9 points per gallon, and it's easy to see why your OG is 1.045.
So if you happen to come up short 9 points, just add one pound. But if you need more or less, what then?
Easy. Divide the number of points you need by the number of points yielded from one pound. That is, if you need 5 points, divide 5/9 = ~0.56 pounds. If you need 11 points, 11/9 = ~1.22 pounds.
Easy as 3.14159265....
If you'd prefer not to do the calculations, and you also happen to brew 5 gallons batches, I put together a table that shows the amount of DME you'd need to add in order to get a certain yield.

[1] Daniels, Ray Designing Great Beers (Brewers Publications, 1996) p.31 - Table 5.1
Wednesday, August 29, 2007
Recipes
American Amber Ale
English Mild
Golden Ale
Nekromyces (Russian Imperial Stout fermented with 3 yeast strains)
Sunday, August 26, 2007
Technical Tuesday: Minerals
Just an announcement this week. I'd like to say that over the course of the next several weeks, I'll be discussing the major minerals important to brewing. It begins next week with Sodium. At the end, I'll explain how to produce a brewing water to your liking from the water that you have.
Item the second:
I harvested just short of three ounces of wet hops today. I grew some Centennial in the backyard over the summer. They look and smell great!
Item the third:
I will be creating a permanent page where you can access my recipes in .xls format. More on that tomorrow.
Yet One More BrewCalc Update
Enjoy!
Thursday, August 23, 2007
BrewCalc Update
It now has a total of five worksheets. The newest worksheet calculates a pitching rate based on George Fix's pitching rate numbers from An Analysis of Brewing Techniques. It provides pitching rates for both ales and lagers. If you want to see the math at work here, check out Jamil's page on Proper Yeast Pitching Rates. It's quite straightforward.
Additionally, there are now a few cells that are automatically populated by figures determined on the first sheet "OG, IBUs, ABV%, etc." This way you don't have to enter certain figures redundantly. Since batch size is a variable that is used in many of the calculations, BrewCalc will now use the figure provided on the first worksheet to determine a host of other numbers. You won't need to go back and forth to change this yourself. This should make it a bit easier to work with.
Wednesday, August 22, 2007
Calculations on the Go
1.) Theoretical Extract in Points per Gallon (TE)
2.) Gallons of Finished Beer (GF)
3.) Typical Extract Efficiency (EE)
4.) Pounds of Grain Mashed (PG)
I tend to use Maris Otter or a slightly lighter British 2-row as my base. It is probably safe to assume that the theoretical yield of this malt is 36 points per gallon. I also know that I do 5 gallon batches, my apparent attenuation floats around 76%, and my typical extract efficiency is 67%.
First thing you're going to do is essentially just figure out your GUs for the batch. That's easy enough.
[(TE*PG)(EE)]/GF or [(36*10)(.67]/5 = 48.24.
We can just round to 48.
This tells you that if you mashed 10 pounds of grain, you could expect a wort of about 1.048 in five gallons.
Now, if we divide pounds of grain by 48, we get a number that can help us figure quickly determine approximate OGs for batches with more or less malt. 10/48 = 0.208333..., but we can call it 0.208, or even 0.21 if you'd like.
If I divide the pounds of malt that I intend to mash, I can get a good idea of what sort of OG my wort will have. 10/.208 = ~48. 8/.208 = ~38.5. 16/.208 = ~77. That is, mashing 8 pounds of malt will yiled an approximate OG of 1.039 in 5 gallons. Mashing 16 will yield an approximate OG of 1.077 in five gallons.
You could also use the calculation to see determine approximately how many pounds of base grain you'll need for an OG of, say, 1.094. Just multiply your desired OG in GUs by your number. Again, in my case this is is 0.208. 0.208*94= ~19.6. So you'll need about 19.6 pounds to get an OG of 1.094 in five gallons.
Of course, this only works well if things in your system stay relatively the same. But there's nothing keeping you from having numbers for Pils malt, or Munich, etc. if you use those grains frequently. Just a little trick to help give you an idea of what you're OG will be like for a certain amount of grain without doing all of the other calculations.Tuesday, August 21, 2007
Technical Tuesday Off Week
In the meantime, why don't you take some time to catch up on the older columns? You could also spend some time reading Lew Bryson's Seen Through A Glass. That's always a good time, especially if you live in or around Philadelphia.
How about a podcast? Basic Brewing, The Jamil Show, and The Sunday Session are three of my favorites.
Maybe consider becoming a beer judge, and AHA member, or checking out the Mr. Wizard column on BYO. Point is, you'll get by. See you next week!
Monday, August 20, 2007
Did I Say Three Worksheets?
It's entitled "Required Water Calculations." Not so surprisingly, it calculates the amount of water that you need for your all grain brewing based on a few parameters. In particular, those are: batch size, pounds of grain mashed, evaporation rate, boil time, and water lost to dead space.
I've also included cells that calculate approximate amounts of yield from the first runnings, as well as how much spare water you'll require to achieve the proper pre-boil volume in your kettle.
Cheers!
BrewCalc
Friday, August 17, 2007
BrewCalc Update
Feel free to send feedback to mashematician [at] gmail [dot] com.
Thursday, August 16, 2007
TBN + First Wort Gravity
3 hectoliters = ~ 79.2 gallons, or ~317 quarts.
First wort gravity = (3 hl/100 kg malt)(20 Plato)/(3.2 hl/100 kg malt)
First wort gravity = 18.75 Plato
Long story short - here's an ultra-simple calculation that you can do to figure this out without exploding your brain:
.6/(X/48)
Replace X with your quarts per pound ratio (1.5, for example), and it will spit out the gravity of your first runnings in degrees Plato. Ta da!
In Excel speak, =SUM(0.6)/(B1/48)
Monday, August 13, 2007
Technical Tuesday: Extract Efficiency
10 lbs. 2-row Pale Malt
2 ozs. Your favorite Noble Hop
Your favorite Ale Strain
You've brewed all grain once or twice before. You get the basic idea of the process, but you're not sure exactly what to expect to get OG-wise from your ten pounds of grain. You also know that variations in gravity while boiling can affect IBU utilization, and you don't want to be far off the mark. After all, this is a beer known for its subtle hopping. How can you figure out what percentage of extract you'll get from your mash?
When brewers talk about achieving a certain extract efficiency, they're talking about the amount of sugar that they coaxed out of the grain expressed as a percentage of the theoretical yield. The theoretical yield differs dependent upon the type of grain being discussed. Pale malt, Munich, Crystal, Roasted, etc. all have different theoretical yields. Luckily, there are tables that contain this information available - some are even free! [Table by Mike Uchima] We'll get back to this in a second. For right now, let's continue on with the example.
You've mashed, sparged, and collected your wort. After correcting for temperature and volume, you determine that you have a wort that will turn out to be around 1.048 in five gallons. You know that this number can be expressed in Gravity Units (GUs) as 48. Since you have five gallons, each gallon containing 48 GUs, you know that the total gravity units of your wort is 240. Since you know that the theoretical yield of two-row is 37 points per gallon, per pound of malt, you would calculate the following:
[GU*Lbs. Malt]/Gallons or, [37*10]/5 = 74. That is, if you extracted 100% of the sugars possible, your wort would have an OG of 1.074. However, it has an OG of 1.048. To determine the percentage of the sugars that you extracted, just divide Actual Extract/Potential Extract. That is, 48/74 = 64.86... ~65%. So, your system has an extract efficiency of ~65!
Of course, few recipes are this simple. Let's look at a slightly more complicated grain bill in order to calculate your efficiency.
8 lbs.2-row Pale (37 ppg)
1 lb. Munich (German) (37 ppg)
0.5 lb. Crystal 40L (37 ppg)
0.25 Chocolate 350L (30 ppg)
Since the system is known to yield around 65% efficency, we can use this to determine the resultant OG. To do this calculation by hand, you would multiply the theoretical extract by the weight of each grain used, multiply by the percentage of extract efficiency, add them all, and divide the number of gallons. It looks something like this:
T=Theoretical Extract
W=Weight of Grains
P=Percentage of Extract Efficiency
G=Total Gallons
((T1*W1*P)+(T2*W2*P)+(T3*W3*P)+(T4*W4*P))/G
For reference, you may want to download small spreadsheet that I put together to show this all in action. You can find it here. As you can see, the only big calculation here is just the calculation given above. It is the contents of cell D2. It reads:
=SUM((B2*37*C2)+(B3*37*C2)+(B4*34*C2)+(B5*30*C2))/C5
That's just the calculation above written for Excel. Armed with this knowledge, you should be able to make cells for all sorts of different fermentables, assuming that you know their theoretical yield. You can use the Uchima table linked to above, or pick up Daniels' Designing Great Beers. It is a book that is quite worthwhile, even if you already understand extract efficiency.
It is worth noting that some fermentables aren't affected by theoretical yield. Malt extracts, for example. Their efficiency is always at 100% since their fermentable sugars don't need to be extracted in any way - they're ready to go. Percentages of extract efficiency only apply to sources that you'll be extracting sugars from after all.
Go forth and be efficient!
Mashematician - Take One
 I ended up employing the design described here. My outlet assembly is a bit more straightforward (read: simplistic) than in the plans, but it's been working well so far. If it has been leaking, it's probably been doing so into the insulation itself. (That is, leaking into the space between the two plastic walls.) I plan on installing a better-sealed outlet in the near future, but this seems to work well enough for now. It's a work in progress after all.
I ended up employing the design described here. My outlet assembly is a bit more straightforward (read: simplistic) than in the plans, but it's been working well so far. If it has been leaking, it's probably been doing so into the insulation itself. (That is, leaking into the space between the two plastic walls.) I plan on installing a better-sealed outlet in the near future, but this seems to work well enough for now. It's a work in progress after all.Here's a shot of me recirculating some wort. It ran off quite clear after vorlaufing about 5 liters or so.
 Lastly, here's a shot of the outdoor burner hard at work.
Lastly, here's a shot of the outdoor burner hard at work.
I concluded that I achieved an extract efficiency of about 67%. It isn't exactly what I'd wanted, but I think that I can improve it by sparging more. As things are, I usually end up on the low side for volume in the fermeter. This would seem to indicate that I should probably continue to collect wort. I recently invested in some high tech pH measuring equipment (okay, it's actually just litmus paper), so I can periodically check the pH of the runoff to ensure that I don't take it down too low.
Speaking of extract efficiency, you may be wondering how I calculated this number. It just so happens that I'll be explaning what extract efficiency is, how to calculate it, and how to put it into a spreadsheet tomorrow for Technical Tuesday.
Finally, I got some awesome birthday gifts from the lovely Vivian as well! Not only did she bring back some very delicious and very interesting beers from her recent trip to Scotland, she gave me a shirt from Valhalla Brewery, a copy of The Homebrewer's Garden (now I can now when to harvest all those Centennial hops growing in my backyard!), and a copy of Sacred Herbal and Healing Beers: The Secrets of Ancient Fermentation. I started reading the latter last night and am enjoying it already. Needless to say, I always appreciate it when I have more to read, especially about beer! Thanks Viv!
Thursday, August 9, 2007
Do Make Say Think
Granted, I'm not alone. I know many people who do this - I'm not making any profound psychological claim about humanity, just noting for my own reference. (See? I did it again.)
The point is, this has been a great space to use to do this sort of thing. It's a matter of forcing myself to organize jumbled and confused ideas and put them down in a way that makes sense. For some things, it's just a matter of repetition. I know that (OG-FG)*131 = ABV% because I've used that calculation hundreds of times. It's just in my head now. So, I'm going to make a concerted effort to do this with other things as well. That brings us to the portion of the blog where I actually do stuff.
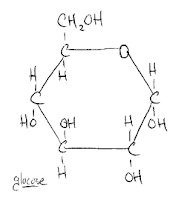 So there's glucose! Not that hard at all. This now makes it much easier to imagine what maltose looks like. Why? Because maltose is two glucose molecules. (Of course, there's a specific location and way in which they share a bond, but I'm not going to get into that right now.) Simply put, I now have a point of reference. I also now have recourse to go out and learn more about the constituent parts of glucose. For example, what's the deal with the CH2OH? I don't know yet, but rest assured that I'll share are soon as I find out.
So there's glucose! Not that hard at all. This now makes it much easier to imagine what maltose looks like. Why? Because maltose is two glucose molecules. (Of course, there's a specific location and way in which they share a bond, but I'm not going to get into that right now.) Simply put, I now have a point of reference. I also now have recourse to go out and learn more about the constituent parts of glucose. For example, what's the deal with the CH2OH? I don't know yet, but rest assured that I'll share are soon as I find out.As a second order of business, I'd like to mention that I recently improved the spreadsheet that I'd first posted about here. It is available for download for free. I have added a calculator to determine the proper temperature for your strike water, as well as putting in space for you to note the types/amounts of fermentables that you used. There's also space for you to note which yeast strain you used. Eventually, I'm going to embed a pitching rate calculator in there. I am also going to attempt to put the recipes I post into this spreadsheet and have them available for download. Here's the recipe I'm planning on brewing tomorrow: Calibration Brew. I'm sure I'll be posting at some point over the weekend as to how that session went, especially considering that it will be my first time using my newly constructed mash/lauter tun.
Lastly, I started tagging entries today. So...that's a thing.
That's it for today. Happy brewing all!
Wednesday, August 8, 2007
SLOM
Anyway, that's probably not very exciting unless you're me or someone who's going to drink some...eventually. What is exciting is that I've found 34oz. Grolsch-style bottles for $2 a piece. Oddly enough, they can be found at Ikea. I give you, SLOM:
 That's pretty cool! You could bottle an entire batch in around 19 bottles if you really wanted to. Granted, they're clear, but if you're not storing your homebrew in dark conditions anyway, I'm not really sure what you're doing with it.
That's pretty cool! You could bottle an entire batch in around 19 bottles if you really wanted to. Granted, they're clear, but if you're not storing your homebrew in dark conditions anyway, I'm not really sure what you're doing with it.I look forward to putting a few big bottles of this stuff away for at least six months. Hopefully I can be patient enough to make it a year. Here's hoping!
Tuesday, August 7, 2007
Technical Tuesday: Mash Thickness, Pt.2
Consider the following from a BYO article by Tom Flores, brewmaster of Brewer's Alley:
"High mash temperatures favor a less fermentable wort because alpha-amylase is a lot more stable than beta-amylase is at higher temperatures. This means that there will be less production of maltose as the activity of beta-amylase diminishes. It is hard to say that beta-amylase activity will be expected to drop off at a particular temperature, because the thickness will determine what temperature activates maximum beta-amylase activity. Thicker mashes tend to retain more beta-amylase activity at high mash temperatures than do thin mashes. This is because beta-amylase is more stable when joined with its substrate than when it is not.
Because beta-amylase encounters substrate less frequently in a thin mash, there is more opportunity for it to be destabilized and inactivated."[1]
If beta-amylase has more of an opportunity to be destabilized and inactivated in a thin mash, why does Noonan write that a thin mash tends to increase maltose production? The two seem to conflict. Is this a function of the fact that the author included the caveat "at high temperatures?" My guess is that this is the case. The quoted optimal range for Beta-amylase activity is substantially lower than that of Alpha-Amylase. Palmer notes that the range for Beta- is between 131-150F, while Alpha- is between 154-162F.[2] If one were doing a mash outside of the optimal range of Beta-amylase, it would stand to reason that a thick mash would be conducted. Why? Just as Flores notes, it tends to destabilize when in further proximity from its substrate. It's already working overtime since it's out of its optimal temperature range. A thinner mash will just increase the work it has to do by making it "seek out" starches to break down.
This would also explain why Noonan writes that "a thick mash...induces the greatest overall extraction."[3] Yes, it may end up being a more dextrinous wort due to the high mash temperatures, but the total end product will be of a greater extract as both Alpha- and Beta-Amylase will be working in concert with one another.
I suppose this is just one more way in which brewing is a balancing act. Mash time, temperature, thickness, pH, etc. are not isolated phenomena. They all work in conjuction and, so it may seem to a new brewer, can even work against one another in some situations. As always, these things will also depend upon the type of system you use as well. Perhaps this is why I'm having a difficult time finding hard and fast rules. That being said, there is still much research to be done.
I still haven't quite mastered the Technical Tuesday portion of this blog, though I hope it's improving.
References:
[1] Flores, Tom Managing Mash Thickness (accessed 8-8-07)
[2] Palmer, John How to Brew (accessed 8-8-07)
[3] Noonan, Gregory New Brewing Lager Beer (Brewers Publications, 1996) p. 140-1
Monday, August 6, 2007
Quick MLT Update
My design is now more reminiscent of the plans found here.
Friday, August 3, 2007
MLT Construction Update

However, I have had no luck finding a 1/2 inch diameter CPVC four-way tee (as would be located at the center of the bottom row of tees). Home Depot has't had them. I called four different local hardware places - nada. I guess I'll just have to make do with what I have for right now. No worries. After all, I still have yet to see what I can squeeze out of the current setup.
I plan to test my new toy later on this week. Here's what I have planned:
Calibration Brew:
Fermentables:
10 lbs. Maris Otter
.5 lb. CaraFoam
Hops:
Centennial @ 60, 10, 5, 1 mins.
Yeast:
Safale S-04
Mash at 153 for 60-75 min.
I'm very interested to see what I can eek out of 10 lbs. of Maris Otter. I can probably expect an OG of 1.045-1.056 in 5 gallons. (Those numbers correlate to 60% and 75% extract efficiency, respectively.)
Lastly, I happened upon this page today. I think this design is pretty clever. Check it out!
Wednesday, August 1, 2007
A Farewell + The Mysteries of Head Retention
Nothing particularly new to report about my beers. I've been pretty content with the way that they've been coming out. I can't wait to build my new mash/lauter tun. I should be starting that one this evening. Hopefully that will help me solve my extract efficiency issue. The only outstanding issue I've had beyond that is achieving satisfactory head retention. Head retention is something I really do not have my head wrapped around. I know there are many oft-cited ways to improve this, e.g. use dextrine malts, try some wheat malt, cut down on adjunct use, don't use dirty glasses, etc. However, only today did I happen across two new potential sources of my problem: 1.) pH. 2.) fermentation temperatures.
I noticed this today. Miller writes, "Check your beer's pH to make sure it is not abnormally low or high. Finished ales should be in the range of 4.0-4.5. "[1] I can't say I'd ever considered that as a source of poor head retention before. I knew that pH could certainly affect mash extract efficiency, but head retention? All the more reason for me to get some litmus paper and start taking notes.
Miller also mentions fermentation temperatures in the same article. He doesn't say much more about how it might affect head retention, though my somewhat-educated guess is that one would be wary of abnormally high temperatures rather than low ones. My thought is that fusel production negatively affects head retention. While I suspect this is true, my reasoning is not exactly flawless. When I think fusels, I recall that they are sometimes referred to as fusel oils. I immediately associted oils with lipids, and lipids with foam inhibition. I'm going to have to look into this further to see if I'm on the right track, but that's what I have off the top of my head.
I feel as though my technical understanding of brewing has been advancing rapidly these past few months. Certainly obtaining copies of Fix's Principles of Brewing Science, Daniels' Designing Great Beers, and Noonan's New Brewing Lager Beer have helped. Even just writing the Technical Tuesday portion of the blog has been helpful. That was the idea from the beginning: to force me to really get the facts straight before writing about them. Hopefully I'm doing an alright job at clarifying some aspects of the hobby - it's a great one.
References:
[1] Miller, Dave BT - Troubleshooter: Vol. 5, No. 5 (accessed Aug. 1 2007)
Tuesday, July 31, 2007
Quick 'N Dirty Tech Tues: Mash Thickness, Pt. 1
Different thicknesses primarily affect the types of sugars that are produced during the mash. (If you need a quick primer on what to expect of worts of different sugar compositions, look here.)
Here's a quick practical rundown of what you can expect from a thick and thin mashes, respectively. Thick mashes tend to produce a higher proportion of dextrins, which lend a fullness and sweetness to the finished beer.[1] Noonan writes, "A thick mash (less than three-tenths of a gallon of water per pound of malt) induces the greatest overall extraction. A much thinner mash increases the proportion of maltose, and thus wort attenuation."[2] For reference, 3/10 of a gallon is 38.4 fluid ounces, or 1.2 quarts.
Bottom line: A thicker mash will typically result in a more dextrinous wort. A thinner mash, on the other hand, will typically result in a thinner, more highly attenuated wort. As far as numbers are concerned, less than 1 qt/lb. would probably be considered thick. Likewise, more than 1.5 qt./lb. would probably be considered on the thin side.
You'll just have to wait around for Part 2 to learn about the mechanisms responsible for this difference.
References:
[1] Noonan, Gregory New Brewing Lager Beer (Brewers Publications, 1996) pg. 140-1[2] Ibid.
Monday, July 30, 2007
Gallimaufry
My memory was not as blurry as I thought. Noonan does differentiate between Caramel malts and Crystal malts in the following way. He writes that Crystal malts are fully saccharified before kilning (thus the glassy endosperm), whereas Caramel malts are not fully saccharified.[1] After discussing the ways in which the production processes differ, he writes the following:
"Caramel malts were traditionally used by continental lagre brewers, whereas crystal malts were favored by British ale brewers. The distinctive, complex flavors of caramel malts have their place in brewing, but unfortunately, modern maltsters are eschewing the production of crisper-flavored crystal malts in favor of the easier-to-process caramel malts. In fact, most modern maltings no longer make a distinction between caramel and crystal malts."[2]
So...is there a difference? Yes. At least there was. Keep in mind that this text was also written eleven years ago. If trends remained the same, then there may be little to no pragmatic difference between caramel and crystal malts. If that's so, then I'm still not sure what the difference is between say, CaraAroma and a crystal malt of a comparable Lovibond rating. I'll have to do some more research before I finally weigh in on this issue.
Second order of business: I brewed an American Amber Ale on Saturday morning. Here's my recipe:
Fermentables:
Maris Otter 62.5%
Munich Type II 31.25%
UK Crystal 60L 3.125%
CaraAroma 3.125%
OG: ~1.065
Hops:
1 oz. Palisade (9.7% AA) 60 min.
1 oz. Athanum (5.1% AA) 15 min.
.5 oz. Palisade 10 min.
.5 oz. Athanum 10 min.
.5 oz. Athanum 5 min.
.5 oz. Palisade @ flameout
Yeast:
Safale S-04
I ended up knocking this batch out in about 5 hours. That even includes the milling of grains. Seems like the process is becoming more refined. There is still one area that is causing me problems - extract efficiency. I calculate my anticipated OG using 65% as the anticipated efficiency. I mashed 16 lbs. of grain at 154F at a rate of 1.1 quarts per pound for ~75 minutes and came up short by 9 GUs. That's an extract efficiency of about 57%. That's quite poor. I did an iodine test to check for proper starch conversion and the resulting color was a deep red, but certainly not black. (The deep red shouldn't be surprising given my mash temp and the inclusion of some darker crystal malts.)
My problem could have to do with many aspects: mash pH, mash thickness, and lautering. I'm still not entirely prepared to jump into the first two right now, but I will be changing my manifold this week to see if that improves yield at all. I hope to post a picture or two of the fully constructed mash/lauter tun later on this week. I plan on using mostly CPVC.
If you'd like to build a cooler-based mash/lauter tun yourself, I would suggest looking at John Palmer's article on the matter.
IBU Dissolution:
For a few weeks now, I've been looking for an answer as to what the theoretical limit of IBU dissolution is. I have not found an answer yet. Tech Talk yielded no useful results. I've emailed my question to the "Ask the Professor" section of Zymurgy, so hopefully something will come up. I'm interested to know because I hear people talking about beers with 100+ IBUs, and it strikes me that the calculations we use as homebrewers to deteremine IBUs can be, well, just wrong. Sure, they can give us a good idea of how much to add for a bittering addition, but it is unlikely that we actually have 35 IBUs in a beer just because a calculation says so. For one, I'd like to know just where the IBU mark ends.
Tomorrow for Technical Tuesday: Mash Thickness.
References:
[1] Noonan, Gregory New Brewing Lager Beer (Brewers Publications, 1996)
[2] Ibid.
Thursday, July 19, 2007
Meeting Success + Recipe Possibilities
There was talk of putting together a competition in early-mid November. Now that I've been more interested in competing, the prospect has me excited. More on that as things progress.
Leaving for BCTC tomorrow! I'd emailed Offshore Ale a day or so ago to let them know that I'd be pouring for them. Just heard back this morning from Joe Cleinman that I'd be pouring with him and the head brewer Matt Steinberg. I look forward to hanging out and pouring some great beers.
Lastly, I'm looking for suggestions. I have a bunch of grains around and I can't decide what to make with them. Here's what I have lying around:
10 lbs. Maris Otter
5 lbs. Munich Type II
.5 lb. CaraAroma
.5 lb. British Crystal 60L
.5 lb. Brown Malt
.5 lb. Carafa I (Dehusked)
I also have 2 ozs. of Palisade (9.7%AA) and some Safale S-04.
I'm thinking I could go any of the following routes: American Amber, American Pale Ale, American Brown, or an Imperial Brown Ale. I'm sort of leaning towards the last option as I haven't made anything much over 1.055 in the past few brews. Let me know what you think.
Wednesday, July 18, 2007
No Tech Tues? + HOPS Meeting
Anyway, this topic is being tabled until next week. The whole basis for this entry is a hazy recollection of a discussion on the issue by Noonan in New Brewing Lager Beer, so I'll need to take a look at that before I try my hand at this one again.
Lastly, I'll be heading over to the HOPS meeting at Home Sweet Homebrew tonight around 6:30-7pm. I'll be taking some Golden Ale and some APA. Looking forward to some feedback from other brewers. That's something I could use more of.
Monday, July 16, 2007
Things to Come
Then, on Friday, it's off to Ommegang! Woo!
Thursday, July 12, 2007
More IBU Talk + English Mild Recipe
Back to our first example. In Schedule #1, it is probably safe to say that the majority of the bitterness will come from the addition at 60 mins (66 IBUs). So if we set up a system whereby the IBU contributions are expressed as ratios, we'd probably need three.
1.) Bitterness:Flavor
2.) Bitterness:Aroma
3.) Flavor:Aroma
So 1.) might look like 66:18, or 1:3.6. 2 might look like 66:.5, or 1:132. 3 might look like 18:.5, or 1:36.
Is this helpful? Eh, I don't know. It seems to be more trouble than its worth. The trouble becomes more apparent when we talk about beers like the one I posited in Schedule #2. Where does flavor end and aroma begin. If I hop a wort for 2.5 minutes, is that an aroma addition or a flavor addition? Should this depend on the IBU contribution of the particular addition? If so, how?
I'm not sure that this would be very useful, especially for beginning brewers. And, as Bouckaert would surely point out, this still doesn't say much more about flavor.
It's probably okay to ditch this idea, or at least table it for now.
Recipe:
I brewed an English Mild last week for the first time! It's not ready yet, but I think I'm in love with the style already. Here are some reasons: 1.) Fermentation was complete in all of five days. 2.) It cost me less than $20 for all the ingredients to make 5 gallons. 3.) The ABV is sufficiently low such that I could just nurse it all day. 4.) It's tasting really nice already! This may just become the house beer.
Fermentables:
5 lbs. Mild Malt (~72%)
.5 lb. British Crystal 60L (~7%)
.5 lb. Brown Malt (~7%)
.5 lb. CaraAroma 120-150L (~7%)
.5 lb. Carafa I (Dehusked) (~7%)
Hops:
.75 ozs. East Kent Goldings (6.2% AA) Just a bittering addition
Yeast:
Safale S-04 (Quickly becoming one of my favorite yeasts)
Mashed at 155F for about 70 minutes.
OG: ~1.045
FG: ~1.011
ABV: ~4.5%
I still seem to have achieved close to 75% AA despite my inclusion of such a high percentage of specialty malts and a high mash temp. I think I may have to mash a bit higher next time to get a more dextrinous wort.
I'm really excited for this beer. I've been on a low alcohol beer kick as of late, and I think this will be one of the best so far. I intend to compete with this beer when the time comes. I'm also ready to drink it!
Tuesday, July 10, 2007
Some Thoughts on Bouckaert's Thoughts
It's not hard to see where he's going with this. (By the way, if you don't already know Bouckaert's position on styles, you should probably first look here. He rails against them.) He even comments on the inclusion of IBU ranges in style guidelines as "ridiculous." (Listen in at the 35:58 mark.) Why?
Because, as he aptly states, "This is a measurement, a measurement that is not relating to taste. I can make you a 40 IBU beer tasting like 25."
And he's right. There are a great many factors that play into creating the overall taste profile of any given beer. Original gravity, water mineral composition, particular hop choice, hopping schedule, fermentability of the wort, etc. all make significant contributions to how the beer will be perceived in the end. Is the bitterness perceived in a 40 IBU Czech Pilsner comparable to that perceived in a 40 IBU English Barleywine? Of course not.
We can still account for some of the differences here with numbers. The GU:BU ratio that Daniels uses in Designing Great Beers comes to mind. (For a beer of an OG of 1.050 and 50 IBUs, the GU:BU ratio would be 1:1.) However, this still isn't the whole story.
Let's talk about hopping schedules for a moment. Say that I want to make an Imperial IPA hopped exclusively with Centennial. Guidelines state that IBUs for this style can range from 60-100+. Using the Tinseth utilization that I so adore, I decided to run some numbers on potential IBUs of an Imperial IPA with an OG of 1.080. For comparison, here are the results of my two different hopping schedules.
Schedule #1:
2.5 ozs. Centennial (10% AA) @ 60 mins - IBU Contribution = 66 IBUs
1 oz. Centennial (10%AA) @ 15 mins - IBU Contribution = 13 IBUs
1 oz. Centennial (10% AA) @ 5 mins - IBU Contribution = 5 IBUS
.5 oz. Centennial (10% AA) @ 1 min - IBU Contribution = .5 IBUS
Total IBU Contributions = 84.5 IBUs
Schedule #2:
2.5 ozs. Centennial (10% AA) @ 20 mins - IBU Contribution = 40 IBUs
1.5 ozs. Centennial (10% AA) @ 15 mins - IBU Contribution = 20 IBUs
1.5 ozs. Centennial (10% AA) @ 10 mins - IBU Contribution = 14 IBUs
1.5 ozs. Centennial (10% AA) @ 5 mins - IBU Contributions = 8 IBUs
1 oz. Centennial (10% AA) @ 1 min - IBU Contribution = 1 IBU
Total IBU Contributions = 83 IBUs
As far as IBU totals go, these two beers are really close. However, the these beers would undoubtedly taste radically different. Where Schedule 1 would produce a beer with a substantial bitterness, Schedule 2 would not. Instead, Schedule 2 would be an over-the-top hop flavor/aroma apocalypse without the extreme bitterness at the end. Should we adopt a ratio of bittering hops to flavor/aroma hops in order to explain this? I'm not sure. But does that mean that inclusion of IBU ranges in the guidelines are "ridiculous"? Perhaps not.
I will say, even as a BJCP judge, that I do not think the guidelines are meant in this way. They are a tool. They can be helpful when designing a recipe for the first time, especially when brewing a style that one has not had the opportunity to try before. I recently brewed a British Mild. As these beers are primarily cask-conditioned real ales served on draught in England, I've never had one. To my knowledge, I've never had an American version of one either. But seeing that they tend to fall in the 10-25 IBU range is helpful - especially since I know that it is not a style where late hopping additions are commonplace. So when I'm doing my calculations, I can take that into account. Later on if I want to brew a Mild dry-hopped with Amarillo, no one's stopping me! However, to get the idea of how they've historically tasted, it behooves me to use the guidelines.
In the end, I appreciate where Bouckaert's coming from. If beer drinkers aren't willing to discuss the possible merits of a given beer becuse it doesn't fall into an established category, then we have a problem. Also, no amount of number crunching will improve how the beer comes together in the glass - that's a product of, as Bouckaert puts it, the knowledge, experience, and creativity of the brewer. That being said, I also think that guidelines can be very useful, especially for those who are new to beer. They succinctly explain many different types of beer and enable the brewer to recreate them, even if the brewer has little experience with the style.
Technical Tuesday: Gravity Calculations for Partial Boils
Let's say you intend to brew five gallons of an American Pale Ale with an original gravity of 1.050. According to Ray Daniels' Designing Great Beers, you would need about 6.6 pounds of LME to achieve this gravity.
Daniels notes that the extract potential of 1 lb. of liquid extract in 1 gallon of water yields between and OG of 1.037 - 1.039.[1]
If we take the average (1.038) and express it in Gravity Units (GUs), we can calculate the following way:
First multiply 50 (target GUs) by 5 gallons. The total gravity units that we're shooting for will be 50 x 5 = 250 GUs.
Then divide the total GUs by the GU potential of the extract. 250/38 = ~6.6.
There you are. ~6.6 pounds is how much you'll need to get an OG of 1.050 in five gallons.
If doing a full-wort boil, you could calculate the hopping rate using 1.050 as your OG. But if all of your fermentables are in three gallons, the gravity of your wort will be higher. Subsequently, hop utilization will go down and you won't be able to extract as much iso-alpha acid from your hops. Since an APA is a fairly bitter beer, you're going to want to adjust your hopping rates to get the desired amount of bitterness.
We can use the following formula to determine what the OG of a given volume would be like assuming it will be 1.050 at 5 gallons. This formula is also courtesy of Daniels.
[GU(beg.) x Volume(beg.) ] / Volume(end) = GU(end) [2]
For example - (60 x 6) / 5 = 72
This formula is helpful for doing things the other way around, that is, figuring out OGs after evaporation. However, we want to solve for GU(beg.), not GU(end). After all, we already know what GU(end) will be...1.050!
In order to go the other way, we use this formula:
[GU(end) x Volume(end)] / Volume(beg.) = GU(beg.) So...
[50 x 5] / 3 = 83.3 - We can round to 83. That is, the OG of your wort is 1.083.
As you can see, that's a big difference in OG of the wort you're boiling!
References:
[1] Daniels, Ray Designing Great Beers (Brewers Publications, 2000) pg. 31 - Table 5.1
[2] Daniels, Ray Designing Great Beers (Brewers Publications, 2000) pg. 36
Friday, July 6, 2007
BJCP Happenings + Yards Update
In Yards news, I got and email from Bill Barton of Yards today indicating that they are currently limiting work that they do on the weekends. However, he also mentioned that as they expand, they may be looking for some extra hands. Hey, I'm just glad to hear back so quickly. Hopefully something will happen with them in the near future. I dig their beers a bunch.
Now on to the weekend...
P.S. A mere two weeks until Ommegang's BCTC!
Thursday, July 5, 2007
Giving It A Shot
In said email I mentioned my desire to work in the craft brewing industry and volunteered my services on the weekends.
I'll keep you posted on what happens from here on out.
Tuesday, July 3, 2007
A Perhaps Not-as-Technical Tuesday: Dry Yeast
The first topic will be Glycogen reserves. Glycogen is for animals what Amylopectin is for plants. In yeast, glycogen reserves serve to maintain life up until the point that glucose uptake from wort is possible. Producers of dry yeast (e.g. Danstar, Fermentis, etc.) take special care to make sure that their yeasts have adequate glycogen reserves to improve their survival rates. Interestingly, this is why it is often not advised that one make a starter with dry yeast. Making a starter uses up the glycogen reserves that the producer had included.
Rehydration is also somewhat related. If you've ever pitched dry yeast straight into a wort without rehydrating beforehand, you probably haven't encountered any problems. But if your beers are turning out fine, why bother with this mysterious hydration business?
In the first few seconds that your yeast is pitched into its new environment, its cell walls are highly permeable. Sugars, hop derivatives, and a host of other wort constituents are capable of killing the yeast at this point as there is no mechanism in place to filter out harmful compounds. Water is the preferred environment for conducting rehydration. See here.
So why is it that your fermentations are proceeding just fine sans rehydration? Easy. The sheer number of cells available in modern dry yeast is quite high. Apparently high enough that the deleterious effects of pitching without rehydrating doesn't seem to cause a huge issue. However, given the small amount of effort necessary to properly rehydrate yeast, there doesn't seem to be reason enough not to. Of course, you could just increase your pitching rate, but don't think that having a bunch of dead yeast in your fermenter is doing wonders for your beer.
By the by, there are some succinct rehydration instructions available here.
Monday, July 2, 2007
Peter Bouckaert!
As though its return wasn't exciting enough, this week's show is Peter Bouckaert's Keynote address from this year's NHC. You can (and should) listen to it here.
So, so good.
Tomorrow for Technical Tuesday - Dried Yeast.
Thursday, June 28, 2007
Aspirations
I've been looking into both the Distance Education programs at the Siebel Institute and the American Brewers Guild. I realize that both are respected, but I'm concerned about putting a lot of time, money, and effort into something that will not help me come any closer to brewing professionally. I really need some good advice on this one.
These things being said, I also really ought to get to more homebrew club meetings and do more judging. I haven't been completely crazy about either so far, though my experience with both has been quite limited. I should also start competing. I know that they're all good things to do, so I'll make it happen.
In other news, I've been very good about keeping my Belgian Red around. I brewed it some time in late May/early June and decided that I would hang on to half a case for a while. I duct-taped it to keep my grubby paws off it and put on a note that it is not to be opened until August 1st. Well, that's not quite going to happen as I intend to bring some to this year's Ommegang Fest to share with others after the fest.
Here's the Belgian Red recipe:
Fermentables:
10 lbs. German Pils
1 lb. CaraRed
1 lb. Melanoidin
.5 lbs. Flaked Oats
1 lb. Corn Sugar
Hops:
Premiant and Saaz
(This beer has 2 oz. of Saaz as the finishing hop.)
Yeast:
Safale T-58
It reached a bit over 80% apparent attenuation. ABV ~6.7%.
It's kind of wicked. This was one of the first beers that I by having a concept of what I wanted to beer to taste like and formulating from there. I really wanted a reddish ale with Belgian-esque fruitiness and phenolics and a complementary Saaz character. Overall, I'm pretty pleased with the result. I expect it to be even more pleasant when I open it up once again.


