Wednesday, August 29, 2007
Recipes
American Amber Ale
English Mild
Golden Ale
Nekromyces (Russian Imperial Stout fermented with 3 yeast strains)
Sunday, August 26, 2007
Technical Tuesday: Minerals
Just an announcement this week. I'd like to say that over the course of the next several weeks, I'll be discussing the major minerals important to brewing. It begins next week with Sodium. At the end, I'll explain how to produce a brewing water to your liking from the water that you have.
Item the second:
I harvested just short of three ounces of wet hops today. I grew some Centennial in the backyard over the summer. They look and smell great!
Item the third:
I will be creating a permanent page where you can access my recipes in .xls format. More on that tomorrow.
Yet One More BrewCalc Update
Enjoy!
Thursday, August 23, 2007
BrewCalc Update
It now has a total of five worksheets. The newest worksheet calculates a pitching rate based on George Fix's pitching rate numbers from An Analysis of Brewing Techniques. It provides pitching rates for both ales and lagers. If you want to see the math at work here, check out Jamil's page on Proper Yeast Pitching Rates. It's quite straightforward.
Additionally, there are now a few cells that are automatically populated by figures determined on the first sheet "OG, IBUs, ABV%, etc." This way you don't have to enter certain figures redundantly. Since batch size is a variable that is used in many of the calculations, BrewCalc will now use the figure provided on the first worksheet to determine a host of other numbers. You won't need to go back and forth to change this yourself. This should make it a bit easier to work with.
Wednesday, August 22, 2007
Calculations on the Go
1.) Theoretical Extract in Points per Gallon (TE)
2.) Gallons of Finished Beer (GF)
3.) Typical Extract Efficiency (EE)
4.) Pounds of Grain Mashed (PG)
I tend to use Maris Otter or a slightly lighter British 2-row as my base. It is probably safe to assume that the theoretical yield of this malt is 36 points per gallon. I also know that I do 5 gallon batches, my apparent attenuation floats around 76%, and my typical extract efficiency is 67%.
First thing you're going to do is essentially just figure out your GUs for the batch. That's easy enough.
[(TE*PG)(EE)]/GF or [(36*10)(.67]/5 = 48.24.
We can just round to 48.
This tells you that if you mashed 10 pounds of grain, you could expect a wort of about 1.048 in five gallons.
Now, if we divide pounds of grain by 48, we get a number that can help us figure quickly determine approximate OGs for batches with more or less malt. 10/48 = 0.208333..., but we can call it 0.208, or even 0.21 if you'd like.
If I divide the pounds of malt that I intend to mash, I can get a good idea of what sort of OG my wort will have. 10/.208 = ~48. 8/.208 = ~38.5. 16/.208 = ~77. That is, mashing 8 pounds of malt will yiled an approximate OG of 1.039 in 5 gallons. Mashing 16 will yield an approximate OG of 1.077 in five gallons.
You could also use the calculation to see determine approximately how many pounds of base grain you'll need for an OG of, say, 1.094. Just multiply your desired OG in GUs by your number. Again, in my case this is is 0.208. 0.208*94= ~19.6. So you'll need about 19.6 pounds to get an OG of 1.094 in five gallons.
Of course, this only works well if things in your system stay relatively the same. But there's nothing keeping you from having numbers for Pils malt, or Munich, etc. if you use those grains frequently. Just a little trick to help give you an idea of what you're OG will be like for a certain amount of grain without doing all of the other calculations.Tuesday, August 21, 2007
Technical Tuesday Off Week
In the meantime, why don't you take some time to catch up on the older columns? You could also spend some time reading Lew Bryson's Seen Through A Glass. That's always a good time, especially if you live in or around Philadelphia.
How about a podcast? Basic Brewing, The Jamil Show, and The Sunday Session are three of my favorites.
Maybe consider becoming a beer judge, and AHA member, or checking out the Mr. Wizard column on BYO. Point is, you'll get by. See you next week!
Monday, August 20, 2007
Did I Say Three Worksheets?
It's entitled "Required Water Calculations." Not so surprisingly, it calculates the amount of water that you need for your all grain brewing based on a few parameters. In particular, those are: batch size, pounds of grain mashed, evaporation rate, boil time, and water lost to dead space.
I've also included cells that calculate approximate amounts of yield from the first runnings, as well as how much spare water you'll require to achieve the proper pre-boil volume in your kettle.
Cheers!
BrewCalc
Friday, August 17, 2007
BrewCalc Update
Feel free to send feedback to mashematician [at] gmail [dot] com.
Thursday, August 16, 2007
TBN + First Wort Gravity
3 hectoliters = ~ 79.2 gallons, or ~317 quarts.
First wort gravity = (3 hl/100 kg malt)(20 Plato)/(3.2 hl/100 kg malt)
First wort gravity = 18.75 Plato
Long story short - here's an ultra-simple calculation that you can do to figure this out without exploding your brain:
.6/(X/48)
Replace X with your quarts per pound ratio (1.5, for example), and it will spit out the gravity of your first runnings in degrees Plato. Ta da!
In Excel speak, =SUM(0.6)/(B1/48)
Monday, August 13, 2007
Technical Tuesday: Extract Efficiency
10 lbs. 2-row Pale Malt
2 ozs. Your favorite Noble Hop
Your favorite Ale Strain
You've brewed all grain once or twice before. You get the basic idea of the process, but you're not sure exactly what to expect to get OG-wise from your ten pounds of grain. You also know that variations in gravity while boiling can affect IBU utilization, and you don't want to be far off the mark. After all, this is a beer known for its subtle hopping. How can you figure out what percentage of extract you'll get from your mash?
When brewers talk about achieving a certain extract efficiency, they're talking about the amount of sugar that they coaxed out of the grain expressed as a percentage of the theoretical yield. The theoretical yield differs dependent upon the type of grain being discussed. Pale malt, Munich, Crystal, Roasted, etc. all have different theoretical yields. Luckily, there are tables that contain this information available - some are even free! [Table by Mike Uchima] We'll get back to this in a second. For right now, let's continue on with the example.
You've mashed, sparged, and collected your wort. After correcting for temperature and volume, you determine that you have a wort that will turn out to be around 1.048 in five gallons. You know that this number can be expressed in Gravity Units (GUs) as 48. Since you have five gallons, each gallon containing 48 GUs, you know that the total gravity units of your wort is 240. Since you know that the theoretical yield of two-row is 37 points per gallon, per pound of malt, you would calculate the following:
[GU*Lbs. Malt]/Gallons or, [37*10]/5 = 74. That is, if you extracted 100% of the sugars possible, your wort would have an OG of 1.074. However, it has an OG of 1.048. To determine the percentage of the sugars that you extracted, just divide Actual Extract/Potential Extract. That is, 48/74 = 64.86... ~65%. So, your system has an extract efficiency of ~65!
Of course, few recipes are this simple. Let's look at a slightly more complicated grain bill in order to calculate your efficiency.
8 lbs.2-row Pale (37 ppg)
1 lb. Munich (German) (37 ppg)
0.5 lb. Crystal 40L (37 ppg)
0.25 Chocolate 350L (30 ppg)
Since the system is known to yield around 65% efficency, we can use this to determine the resultant OG. To do this calculation by hand, you would multiply the theoretical extract by the weight of each grain used, multiply by the percentage of extract efficiency, add them all, and divide the number of gallons. It looks something like this:
T=Theoretical Extract
W=Weight of Grains
P=Percentage of Extract Efficiency
G=Total Gallons
((T1*W1*P)+(T2*W2*P)+(T3*W3*P)+(T4*W4*P))/G
For reference, you may want to download small spreadsheet that I put together to show this all in action. You can find it here. As you can see, the only big calculation here is just the calculation given above. It is the contents of cell D2. It reads:
=SUM((B2*37*C2)+(B3*37*C2)+(B4*34*C2)+(B5*30*C2))/C5
That's just the calculation above written for Excel. Armed with this knowledge, you should be able to make cells for all sorts of different fermentables, assuming that you know their theoretical yield. You can use the Uchima table linked to above, or pick up Daniels' Designing Great Beers. It is a book that is quite worthwhile, even if you already understand extract efficiency.
It is worth noting that some fermentables aren't affected by theoretical yield. Malt extracts, for example. Their efficiency is always at 100% since their fermentable sugars don't need to be extracted in any way - they're ready to go. Percentages of extract efficiency only apply to sources that you'll be extracting sugars from after all.
Go forth and be efficient!
Mashematician - Take One
 I ended up employing the design described here. My outlet assembly is a bit more straightforward (read: simplistic) than in the plans, but it's been working well so far. If it has been leaking, it's probably been doing so into the insulation itself. (That is, leaking into the space between the two plastic walls.) I plan on installing a better-sealed outlet in the near future, but this seems to work well enough for now. It's a work in progress after all.
I ended up employing the design described here. My outlet assembly is a bit more straightforward (read: simplistic) than in the plans, but it's been working well so far. If it has been leaking, it's probably been doing so into the insulation itself. (That is, leaking into the space between the two plastic walls.) I plan on installing a better-sealed outlet in the near future, but this seems to work well enough for now. It's a work in progress after all.Here's a shot of me recirculating some wort. It ran off quite clear after vorlaufing about 5 liters or so.
 Lastly, here's a shot of the outdoor burner hard at work.
Lastly, here's a shot of the outdoor burner hard at work.
I concluded that I achieved an extract efficiency of about 67%. It isn't exactly what I'd wanted, but I think that I can improve it by sparging more. As things are, I usually end up on the low side for volume in the fermeter. This would seem to indicate that I should probably continue to collect wort. I recently invested in some high tech pH measuring equipment (okay, it's actually just litmus paper), so I can periodically check the pH of the runoff to ensure that I don't take it down too low.
Speaking of extract efficiency, you may be wondering how I calculated this number. It just so happens that I'll be explaning what extract efficiency is, how to calculate it, and how to put it into a spreadsheet tomorrow for Technical Tuesday.
Finally, I got some awesome birthday gifts from the lovely Vivian as well! Not only did she bring back some very delicious and very interesting beers from her recent trip to Scotland, she gave me a shirt from Valhalla Brewery, a copy of The Homebrewer's Garden (now I can now when to harvest all those Centennial hops growing in my backyard!), and a copy of Sacred Herbal and Healing Beers: The Secrets of Ancient Fermentation. I started reading the latter last night and am enjoying it already. Needless to say, I always appreciate it when I have more to read, especially about beer! Thanks Viv!
Thursday, August 9, 2007
Do Make Say Think
Granted, I'm not alone. I know many people who do this - I'm not making any profound psychological claim about humanity, just noting for my own reference. (See? I did it again.)
The point is, this has been a great space to use to do this sort of thing. It's a matter of forcing myself to organize jumbled and confused ideas and put them down in a way that makes sense. For some things, it's just a matter of repetition. I know that (OG-FG)*131 = ABV% because I've used that calculation hundreds of times. It's just in my head now. So, I'm going to make a concerted effort to do this with other things as well. That brings us to the portion of the blog where I actually do stuff.
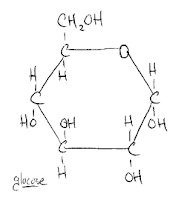 So there's glucose! Not that hard at all. This now makes it much easier to imagine what maltose looks like. Why? Because maltose is two glucose molecules. (Of course, there's a specific location and way in which they share a bond, but I'm not going to get into that right now.) Simply put, I now have a point of reference. I also now have recourse to go out and learn more about the constituent parts of glucose. For example, what's the deal with the CH2OH? I don't know yet, but rest assured that I'll share are soon as I find out.
So there's glucose! Not that hard at all. This now makes it much easier to imagine what maltose looks like. Why? Because maltose is two glucose molecules. (Of course, there's a specific location and way in which they share a bond, but I'm not going to get into that right now.) Simply put, I now have a point of reference. I also now have recourse to go out and learn more about the constituent parts of glucose. For example, what's the deal with the CH2OH? I don't know yet, but rest assured that I'll share are soon as I find out.As a second order of business, I'd like to mention that I recently improved the spreadsheet that I'd first posted about here. It is available for download for free. I have added a calculator to determine the proper temperature for your strike water, as well as putting in space for you to note the types/amounts of fermentables that you used. There's also space for you to note which yeast strain you used. Eventually, I'm going to embed a pitching rate calculator in there. I am also going to attempt to put the recipes I post into this spreadsheet and have them available for download. Here's the recipe I'm planning on brewing tomorrow: Calibration Brew. I'm sure I'll be posting at some point over the weekend as to how that session went, especially considering that it will be my first time using my newly constructed mash/lauter tun.
Lastly, I started tagging entries today. So...that's a thing.
That's it for today. Happy brewing all!
Wednesday, August 8, 2007
SLOM
Anyway, that's probably not very exciting unless you're me or someone who's going to drink some...eventually. What is exciting is that I've found 34oz. Grolsch-style bottles for $2 a piece. Oddly enough, they can be found at Ikea. I give you, SLOM:
 That's pretty cool! You could bottle an entire batch in around 19 bottles if you really wanted to. Granted, they're clear, but if you're not storing your homebrew in dark conditions anyway, I'm not really sure what you're doing with it.
That's pretty cool! You could bottle an entire batch in around 19 bottles if you really wanted to. Granted, they're clear, but if you're not storing your homebrew in dark conditions anyway, I'm not really sure what you're doing with it.I look forward to putting a few big bottles of this stuff away for at least six months. Hopefully I can be patient enough to make it a year. Here's hoping!
Tuesday, August 7, 2007
Technical Tuesday: Mash Thickness, Pt.2
Consider the following from a BYO article by Tom Flores, brewmaster of Brewer's Alley:
"High mash temperatures favor a less fermentable wort because alpha-amylase is a lot more stable than beta-amylase is at higher temperatures. This means that there will be less production of maltose as the activity of beta-amylase diminishes. It is hard to say that beta-amylase activity will be expected to drop off at a particular temperature, because the thickness will determine what temperature activates maximum beta-amylase activity. Thicker mashes tend to retain more beta-amylase activity at high mash temperatures than do thin mashes. This is because beta-amylase is more stable when joined with its substrate than when it is not.
Because beta-amylase encounters substrate less frequently in a thin mash, there is more opportunity for it to be destabilized and inactivated."[1]
If beta-amylase has more of an opportunity to be destabilized and inactivated in a thin mash, why does Noonan write that a thin mash tends to increase maltose production? The two seem to conflict. Is this a function of the fact that the author included the caveat "at high temperatures?" My guess is that this is the case. The quoted optimal range for Beta-amylase activity is substantially lower than that of Alpha-Amylase. Palmer notes that the range for Beta- is between 131-150F, while Alpha- is between 154-162F.[2] If one were doing a mash outside of the optimal range of Beta-amylase, it would stand to reason that a thick mash would be conducted. Why? Just as Flores notes, it tends to destabilize when in further proximity from its substrate. It's already working overtime since it's out of its optimal temperature range. A thinner mash will just increase the work it has to do by making it "seek out" starches to break down.
This would also explain why Noonan writes that "a thick mash...induces the greatest overall extraction."[3] Yes, it may end up being a more dextrinous wort due to the high mash temperatures, but the total end product will be of a greater extract as both Alpha- and Beta-Amylase will be working in concert with one another.
I suppose this is just one more way in which brewing is a balancing act. Mash time, temperature, thickness, pH, etc. are not isolated phenomena. They all work in conjuction and, so it may seem to a new brewer, can even work against one another in some situations. As always, these things will also depend upon the type of system you use as well. Perhaps this is why I'm having a difficult time finding hard and fast rules. That being said, there is still much research to be done.
I still haven't quite mastered the Technical Tuesday portion of this blog, though I hope it's improving.
References:
[1] Flores, Tom Managing Mash Thickness (accessed 8-8-07)
[2] Palmer, John How to Brew (accessed 8-8-07)
[3] Noonan, Gregory New Brewing Lager Beer (Brewers Publications, 1996) p. 140-1
Monday, August 6, 2007
Quick MLT Update
My design is now more reminiscent of the plans found here.
Friday, August 3, 2007
MLT Construction Update

However, I have had no luck finding a 1/2 inch diameter CPVC four-way tee (as would be located at the center of the bottom row of tees). Home Depot has't had them. I called four different local hardware places - nada. I guess I'll just have to make do with what I have for right now. No worries. After all, I still have yet to see what I can squeeze out of the current setup.
I plan to test my new toy later on this week. Here's what I have planned:
Calibration Brew:
Fermentables:
10 lbs. Maris Otter
.5 lb. CaraFoam
Hops:
Centennial @ 60, 10, 5, 1 mins.
Yeast:
Safale S-04
Mash at 153 for 60-75 min.
I'm very interested to see what I can eek out of 10 lbs. of Maris Otter. I can probably expect an OG of 1.045-1.056 in 5 gallons. (Those numbers correlate to 60% and 75% extract efficiency, respectively.)
Lastly, I happened upon this page today. I think this design is pretty clever. Check it out!
Wednesday, August 1, 2007
A Farewell + The Mysteries of Head Retention
Nothing particularly new to report about my beers. I've been pretty content with the way that they've been coming out. I can't wait to build my new mash/lauter tun. I should be starting that one this evening. Hopefully that will help me solve my extract efficiency issue. The only outstanding issue I've had beyond that is achieving satisfactory head retention. Head retention is something I really do not have my head wrapped around. I know there are many oft-cited ways to improve this, e.g. use dextrine malts, try some wheat malt, cut down on adjunct use, don't use dirty glasses, etc. However, only today did I happen across two new potential sources of my problem: 1.) pH. 2.) fermentation temperatures.
I noticed this today. Miller writes, "Check your beer's pH to make sure it is not abnormally low or high. Finished ales should be in the range of 4.0-4.5. "[1] I can't say I'd ever considered that as a source of poor head retention before. I knew that pH could certainly affect mash extract efficiency, but head retention? All the more reason for me to get some litmus paper and start taking notes.
Miller also mentions fermentation temperatures in the same article. He doesn't say much more about how it might affect head retention, though my somewhat-educated guess is that one would be wary of abnormally high temperatures rather than low ones. My thought is that fusel production negatively affects head retention. While I suspect this is true, my reasoning is not exactly flawless. When I think fusels, I recall that they are sometimes referred to as fusel oils. I immediately associted oils with lipids, and lipids with foam inhibition. I'm going to have to look into this further to see if I'm on the right track, but that's what I have off the top of my head.
I feel as though my technical understanding of brewing has been advancing rapidly these past few months. Certainly obtaining copies of Fix's Principles of Brewing Science, Daniels' Designing Great Beers, and Noonan's New Brewing Lager Beer have helped. Even just writing the Technical Tuesday portion of the blog has been helpful. That was the idea from the beginning: to force me to really get the facts straight before writing about them. Hopefully I'm doing an alright job at clarifying some aspects of the hobby - it's a great one.
References:
[1] Miller, Dave BT - Troubleshooter: Vol. 5, No. 5 (accessed Aug. 1 2007)


